REVOLUTIONIZING TARGETED CANCER THERAPY WITH NEXT-GENERATION ANTIBODY-DRUG CONJUGATES (ADCs)
OVERCOMING TOXICITY AND RESISTANCE IN TRADITIONAL ADCs
Antibody-drug conjugates have long offered immense promise as targeted anti-cancer therapies. However traditional ADC technologies still face major shortcomings in dose-limiting systemic toxicity, therapeutic resistance, and suboptimal stability that together undermine efficacy. Our multi-pronged ADC platform is overcoming these intrinsic flaws by pioneering next-generation linker and payload chemistries.
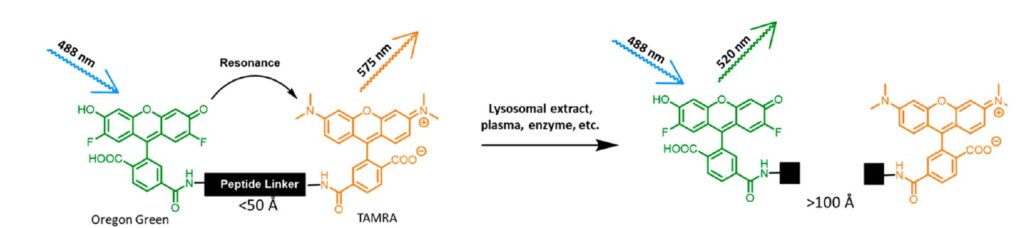

High throughput FRET-based screen to identify novel cleavable linkers
PIONEERING NEW LINKERS: PRECISION ACTIVATION WITH LEGUMAIN SYSTEM
At the core of our ADC technology lies an innovative legumain-activated linker system enabling localized payload release specifically within the tumor microenvironment. Legumain is a cysteine protease upregulated across cancers compared to healthy tissue. Our custom peptide linker incorporates legumain-cleavable sequences that remain stable in circulation but facilitate rapid activation upon exposure to lysosomal legumain following ADC internalization into tumor cells. This controlled, cancer cell-specific activation prevents systemic drug release, increasing localized cytotoxicity at malignant sites while avoiding healthy tissue
ADVANCING NEXT-GENERATION TOP1i WARHEADS
We are optimizing cutting-edge topoisomerase I inhibitor (TOP1i) payloads that drive cancer cell death by overloading native DNA repair processes and generating irreparable DNA double strand breaks. Specifically, we have developed exatecan derivatives coupled to legumain cleavable linkers to release these potent cytotoxins in the lysosomes of tumor cells. While exceptionally cytotoxic, payload resistance mechanisms and dose-limiting toxicity have challenged their clinical success. By targeted tumor delivery, our legumain linker enables circumventing these limitations to finally harness the power of this unique payload class. Moreover, by leveraging vast in-house linker and payload libraries coupled with innovations in synthetic chemistry and bioconjugation techniques, we are continually pioneering new generations of tumor-selective linker-payload systems to enhance targeted delivery and therapeutic action of the ultra-potent cytotoxins
FDA-APPROVED ENHERTU® (TRASTUZUMAB DERUXTECAN)
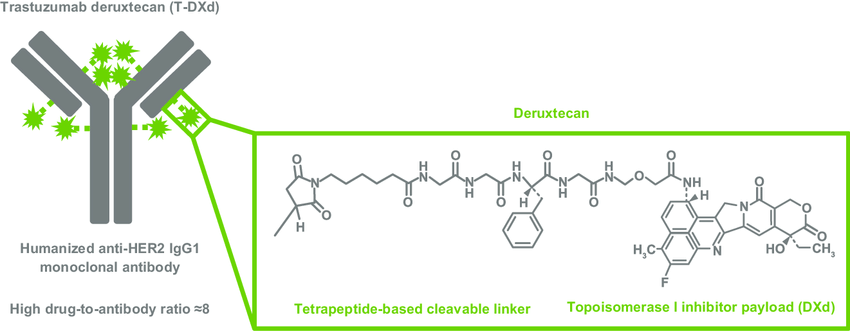
Interstitial lung disease (ILD)/pneumonitis is an important risk for patients treated with T-DXd and must be closely monitored and managed according to recommended guidelines
DRAWBACKS OF CLEAVABLE GGFG LINKERS
• The glycyl-glycyl-phenylalanyl-glycine (GGFG) peptide linker used in ENHERTU® is subject to some degree of premature cathepsin cleavage like val-cit linkers
• Cathepsin heterogeneity and overexpression: Cathepsins like Cathepsin B/L are quite heterogeneous and overexpressed in some tissue types beyond tumors. This could lead to off-target cleavage of the GGFG linker, especially in macrophages, releasing the cytotoxic payload prematurely before reaching the tumor
• Albumin binding variability: While the GGFG linker binds albumin in theory to prevent premature release, the strength and duration of albumin binding may vary depending on environmental conditions in circulation and the tumor microenvironment
DRAWBACKS OF CLEAVABLE VAL-CIT LINKERS
• Ubiquitous expression of cathepsin B/L/S proteases that cleave linker leads to premature release, systemic exposure, and toxicity
• Non-selective activation in blood by circulating proteases degrading linker over time in circulation even before reaching tumor
• Variable linker stability depending on tumor microenvironment protease expression
• Risk of drug resistance if target antigen is downregulated, exposing cytotoxic payload to systemic circulation
• Myelosuppression, which is most likely caused by premature linker cleavage and payload release
REALIZING THE FULL POTENTIAL OF ADCS
By enhancing stability prior to activation, precision tumor targeting, and leveraging next-generation payloads, our ADC platform has consistently demonstrated remarkable preclinical improvements across key metrics of efficacy and safety compared to existing ADC technologies. We are unlocking the long-promised yet elusive potential of antibody-drug conjugates to transform targeted cancer therapy
TWO ADCs DELIVERING TOPOISOMERASE I (TOP1) POISONS (ENHERTU AND TRODELVY) HAVE RECENTLY BEEN FDA-APPROVED FOR HER2- AND TROP2-EXPRESSING SOLID TUMORS
HOW ENHERTU® HER2-DIRECTED ADC WORKS ?
HOW TRODELVY® TROP2-DIRECTED ADC WORKS?
OUR PUBLICATIONS ON ANTIBODY-DRUG CONJUGATES
Selected publications by Dr Borys Shor display the citations in Pubmed
1: Betts AM, Haddish-Berhane N, Tolsma J, Jasper P, King LE, Sun Y, Chakrapani S, Shor B, Boni J, Johnson TR. Preclinical to Clinical Translation of Antibody- Drug Conjugates Using PK/PD Modeling: a Retrospective Analysis of Inotuzumab Ozogamicin. AAPS J. 2016 Sep;18(5):1101-1116. doi: 10.1208/s12248-016-9929-7. Epub 2016 May 19. PMID: 27198897.
2: Shor B, Kahler J, Dougher M, Xu J, Mack M, Rosfjord E, Wang F, Melamud E, Sapra P. Enhanced Antitumor Activity of an Anti-5T4 Antibody-Drug Conjugate in Combination with PI3K/mTOR inhibitors or Taxanes. Clin Cancer Res. 2016 Jan 15;22(2):383-94. doi: 10.1158/1078-0432.CCR-15-1166. Epub 2015 Aug 28. PMID: 26319086.
3. Mack, M., Kahler, J., Shor, B., Ritchie, M., Dougher, M., Sung, M. and Sapra, P. (2016). Lysosomes and Antibody–Drug Conjugates. In Lysosomes: Biology, Diseases, and Therapeutics (eds F.R. Maxfield, J.M. Willard and S. Lu). https://doi.org/10.1002/9781118978320.ch17
4: Shor B, Gerber HP, Sapra P. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Mol Immunol. 2015 Oct;67(2 Pt A):107-16. doi: 10.1016/j.molimm.2014.09.014. Epub 2014 Oct 7. PMID: 25304309.
5: He H, Ratnayake AS, Janso JE, He M, Yang HY, Loganzo F, Shor B, O’Donnell CJ, Koehn FE. Cytotoxic Spliceostatins from Burkholderia sp. and Their Semisynthetic Analogues. J Nat Prod. 2014 Aug 22;77(8):1864-70. doi: 10.1021/np500342m. PMID: 25098528.
6: Sapra P, Shor B. Monoclonal antibody-based therapies in cancer: advances and challenges. Pharmacol Ther. 2013 Jun;138(3):452-69. doi: 10.1016/j.pharmthera.2013.03.004. Epub 2013 Mar 15. PMID: 23507041.
Selected publications by Dr Nathan Tumey: display the citations in Pubmed
1: ValCitGlyPro-dexamethasone antibody conjugates selectively suppress the activation of human monocytes. Howe JM, Fang S, Watts KA, Xu F, Benjamin SR, Tumey LN. RSC Med Chem. 2023 Sep 20;14(11):2348-2357. doi: 10.1039/d3md00336a. eCollection 2023 Nov 15. PMID: 37974960
2: Synthesis and Optimization of 1-Substituted Imidazo[4,5-c]quinoline TLR7 Agonists. DeYoung EG, Howe JM, Fang S, Reddy MM, Handel JP, Gillen Miller JT, Wheeler DR, Tumey LN. ACS Med Chem Lett. 2023 Sep 14;14(10):1358-1368. doi: 10.1021/acsmedchemlett.3c00260. eCollection 2023 Oct 12. PMID: 37849530
3: Visualizing drug-induced lipid accumulation in lysosomes of live cancer cells with stimulated Raman imaging. Yuan Y, Olawode EO, Tumey LN, Lu F. Biomed Opt Express. 2023 May 8;14(6):2551-2564. doi: 10.1364/BOE.487527. eCollection 2023 Jun 1. PMID: 37342714 Free PMC article.
4: Indoloquinoline-Mediated Targeted Downregulation of KRAS through Selective Stabilization of the Mid-Promoter G-Quadruplex Structure. Psaras AM, Carty RK, Miller JT, Tumey LN, Brooks TA. Genes (Basel). 2022 Aug 13;13(8):1440. doi: 10.3390/genes13081440. PMID: 36011352 Free PMC article.
5: Evaluation of an ester-linked immunosuppressive payload: A case study in understanding the stability and cleavability of ester- containing ADC linkers. Jackson CP, Fang S, Benjamin SR, Alayi T, Hathout Y, Gillen SM, Handel JP, Brems BM, Howe JM, Tumey LN. Bioorg Med Chem Lett. 2022 Nov 1;75:128953. doi: 10.1016/j.bmcl.2022.128953. Epub 2022 Sep 1. PMID: 36058468.
6: Design and Characterization of Immune-Stimulating Imidazo[4,5-c]quinoline Antibody-Drug Conjugates. Fang S, Brems BM, Olawode EO, Miller JT, Brooks TA, Tumey LN. Mol Pharm. 2022 Sep 5;19(9):3228-3241. doi: 10.1021/acs.molpharmaceut.2c00392. Epub 2022 Jul 29. PMID: 35904247.
7: Enzyme-Agnostic Lysosomal Screen Identifies New Legumain-Cleavable ADC Linkers. Miller JT, Vitro CN, Fang S, Benjamin SR, Tumey LN. Bioconjug Chem. 2021 Apr 21;32(4):842-858. doi: 10.1021/acs.bioconjchem.1c00124. Epub 2021 Mar 31. PMID: 33788548.
Selected publications by Dr Dhaval Shah: display the citations in Pubmed
1: Development of a generalized pharmacokinetic model to characterize clinical pharmacokinetics of monomethyl auristatin E-based antibody-drug conjugates.
Chang HP, Cheung YK, Liu S, Shah DK. Br J Clin Pharmacol. 2024 Apr 2. doi: 10.1111/bcp.16057. Online ahead of print. PMID: 38566392
2: Investigation of Antibody Pharmacokinetics in the Brain Following Intra-CNS Administration and Development of PBPK Model to Characterize the Data.
Wu S, Chang HY, Chowdhury EA, Huang HW, Shah DK. AAPS J. 2024 Mar 5;26(2):29. doi: 10.1208/s12248-024-00898-7. PMID: 38443635
3: Expansion of platform physiologically-based pharmacokinetic model for monoclonal antibodies towards different preclinical species: cats, sheep, and dogs. Huang HW, Wu S, Chowdhury EA, Shah DK.
J Pharmacokinet Pharmacodyn. 2023 Nov 10. doi: 10.1007/s10928-023-09893-5. Online ahead of print. PMID: 37947924
4: Physiologically Based Pharmacokinetic Modeling to Characterize the Effect of Molecular Charge on Whole-Body Disposition of Monoclonal Antibodies. Liu S, Shah DK. AAPS J. 2023 Apr 28;25(3):48. doi: 10.1208/s12248-023-00812-7. PMID: 37118220.
5: Pharmacokinetics and Pharmacodynamics of Antibody- Drug Conjugates Administered via Subcutaneous and Intratumoral Routes. Chang HP, Le HK, Shah DK. Pharmaceutics. 2023 Apr 3;15(4):1132. doi: 10.3390/pharmaceutics15041132. PMID: 37111619.
6: Development of a Physiologically-Based Pharmacokinetic Model for Whole-Body Disposition of MMAE Containing Antibody- Drug Conjugate in Mice. Chang HP, Li Z, Shah DK. Pharm Res. 2022 Jan;39(1):1-24. doi: 10.1007/s11095-021-03162-1. Epub 2022 Jan 19. PMID: 35044590.
7: Quantitative Evaluation of the Effect of Antigen Expression Level on Antibody-Drug Conjugate Exposure in Solid Tumor. Bussing D, Sharma S, Li Z, Meyer LF, Shah DK. AAPS J. 2021 Apr 15;23(3):56. doi: 10.1208/s12248-021-00584-y. PMID: 33856579.23:
8: Whole-Body Pharmacokinetics and Physiologically Based Pharmacokinetic Model for Monomethyl Auristatin E (MMAE). Chang HP, Cheung YK, Shah DK. J Clin Med. 2021 Mar 23;10(6):1332. doi: 10.3390/jcm10061332. PMID: 33807057 Free PMC article.